
Priming with Sugar
Proper carbonation of beer enhances its quality. The relatively low carbonation of British beers accentuates their maltiness while the higher carbonation of European and American beers gives them a lighter, more spritzy taste. It also brings out the aroma of the hops. An undercarbonated beer is flat and lifeless; an over carbonated beer fills your glass with foam and not beer. Proper carbonation for the style beer you brew is important to its enjoyment. An adequate level of carbonation in also required in order to have a good foam stand.
There are three methods used to carbonate beer: priming with sugar or wort, forced carbonation and the retention of carbon dioxide gas (CO2) generated late in fermentation. Most homebrewers add a measured amount of corn sugar or cane sugar to their bottling bucket to bottle condition their beers. The sugar is consumed by the yeast and CO2 is produced.
Glucose and Sucrose
Glucose is a six-carbon sugar. There are two isomers of glucose — D-glucose and L-glucose. The “D” form is produced and consumed by biological organisms and D-glucose is often called dextrose. D-glucose extracted from corn is often called corn sugar.
Glucose (or dextrose or corn sugar) powder is sold in two different forms — anhydrous glucose and glucose monohydrate, both of which are white powders. Anhydrous glucose is pure glucose. (Anhydrous means “without water.’) Glucose monohydrate is glucose with one molecule of water complexed with each glucose molecule. Nine percent of the weight of glucose monohydrate is due to water and it will not contribute to CO2 production during bottle conditioning. Anhydrous glucose is hygroscopic (it absorbs moisture from the air) and will form “rocks” if not stored in a sealed container.
If you are wondering whether your corn sugar is anhydrous glucose or glucose monohydrate, make a 3 x 3 inch (7.6 x 7.6 cm) tray out of aluminum foil, with 1-inch (2.5-cm) sides. Weigh out about an ounce (28 g) of corn sugar in the tray and put it in your oven at 275 °F (135 °C). This temperature will drive off the complexed water. Remove the tray after 30 minutes and reweigh. Kick your oven temperature up to 300 °F (149 °C), a temperature that will melt the glucose. Reweigh. I found a 10% drop in weight, confirming that my corn sugar is glucose monohydrate.
Sucrose is a two-subunit sugar composed of glucose and fructose (another six-carbon sugar). The major sources of sucrose is sugar cane (cane sugar) and sugar beets (beet sugar). Like glucose, sucrose is white powder. Unlike glucose, sucrose does not form hydrated complexes, nor is it hygroscopic.
From Chemistry to Carbonation
Below this paragraph are the chemical reactions that take place during bottle conditioning. Using these reactions, we can derive an equation to calculate carbonation levels from sugar additions.

Using the information from this chart, and some basic chemical relationships, we can derive the following formula for 5.00-gallon (18.9-L) batches of beer:
(X g glucose) x (88 g CO2/180 g glucose) x (1 mole/44 g CO2) x (22.4 L/mole) x (1/18.9 L beer) = Y volumes of CO2
where X is the weight of (anhydrous) glucose and Y is the level of carbonation. For example, if you add 3.0 oz (85 g) of anhydrous glucose, you would generate 85 x 0.489 x 0.0227 x 22.4 x 0.0529 = 85 x 0.0132 = 1.12 volumes of CO2 in 5.0 gallons (19 L) of beer.
Analogous equations can be derived for glucose monohydrate and sucrose using the information from their chemical reactions. These equations will tell you how many of volumes of CO2 are generated by a given amount of sugar.
If you don’t want to calculate these volumes on your own, click on this link for the carbonation priming charts.
Residual Carbon Dioxide
One must remember that, after fermentation, your beer is saturated with carbon dioxide at atmospheric pressure. Although an ale may seem “flat” when sampled from the fermenter, there is a small amount of gas dissolved in it. Lagers usually have a low, but noticeable, level of carbonation immediately after fermentation.
Beer carbonation tables available in the books I have do not extend to zero gauge pressure (1 atmosphere at sea level). They also do not include fermentation temperatures above 60 °F (16 °C), because they are presented for force carbonation use, and beers — even in England — are not generally served at temperatures higher than this. For the same reason, these tables do not show carbonation levels at atmospheric pressure where many fermentations occur.
However, the data presented can be accurately extended to atmospheric pressure using Henry’s Law, which says that the amount of gas absorbed in a liquid at a given temperature is directly proportional to the pressure of that gas in equilibrium with the liquid. Using Henry’s Law for the carbonation charts and a curve fit to extend the data to higher fermentation temperatures, I developed the graph below.
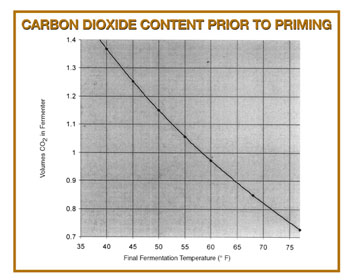
Level of Carbonation in Bottle
To determine the carbonation volumes expected in your final beer, you need to add the priming sugar contribution to the volumes present prior to the addition of the priming sugar. For example, I ferment my ales at about 65 °F (18 °C). This means that I have about 0.89 volumes of CO2 present before priming. If the beer is a British bitter, I would add 2.5 oz. (70 g) of my corn sugar to my bottling bucket, which would add an additional 0.85 volumes of CO2, giving me an expected 1.74 volumes, near the middle of the range shown for British ales.
Keep in mind that, if your beer warms up after fermentation, it will lose CO2. This will not happen instantaneously, though. However, lowering your beer’s temperature will not increase the level of CO2, unless a source of CO2 is present. (Continuing fermentation or CO2 from an outside source — like CO2 cylinder — are the two most likely possibilities.) If you want to accurately estimate your residual amount of carbon dioxide, hold your fermentation temperature constant and add the priming sugar to the beer at this same temperature. You may then warm the beer up for bottle conditioning, if needed.
Priming in Style
Different styles of beer call for different levels of carbonation. The following table outlines the appropriate ranges for various beer styles:
Style & Volumes of CO2
American ales 2.2–3.0
British ales 1.5–2.2
German weizens 2.8–5.1
Belgian ales 2.0–4.5
European Lagers 2.4–2.6
American Lagers 2.5–2.8
Three-Quarters of a Cup?
You may be wondering — do I need to bother with all of this? Can’t I just keep adding 0.75 cups of corn sugar? The answer, of course, is up to you, but here are some facts.
Three quarters of a cup of corn sugar (glucose monohydrate), which weighs around 4.5 oz. (128 g), added to an ale fermented at 70 °F (21 °C) would yield about 2.5 volumes of CO2. However, if you add the same amount to a beer that was fermented and primed at 50 °F (10 °C), you get about 2.9 volumes of CO2.